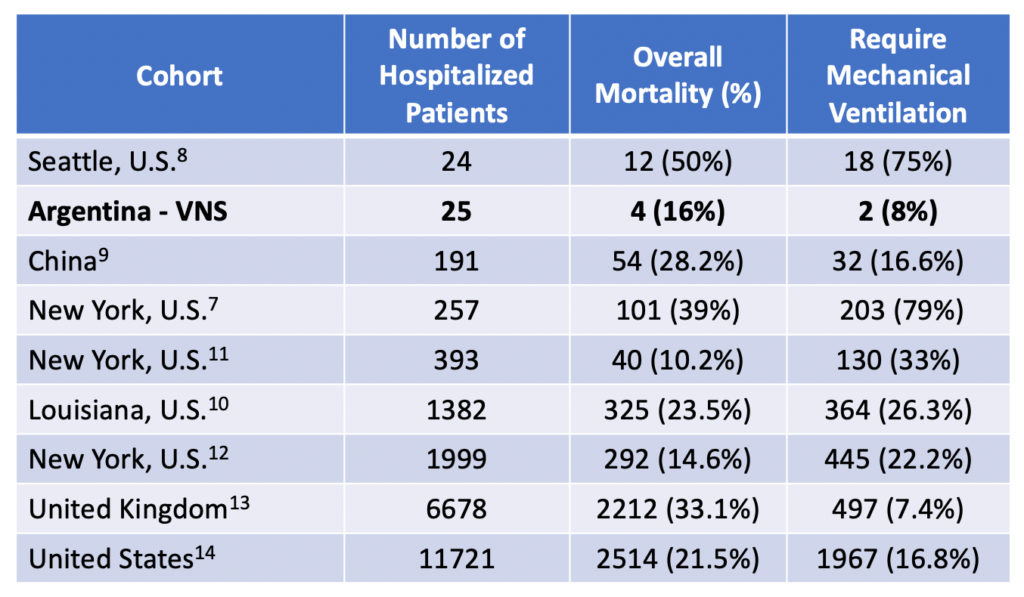
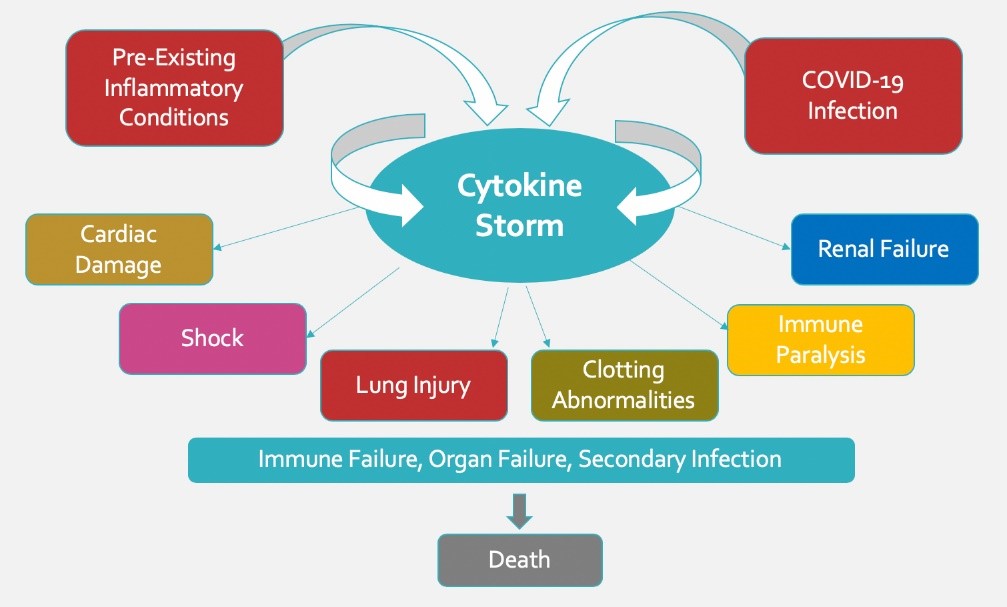
PR: Vagus Nerve Stimulation Associated with Fewer Deaths in Hospitalized COVID-19 Patients, Dec 2, 2020
OVERLAND PARK, KANSAS, UNITED STATES, December 2, 2020 /EINPresswire.com/ — Nemechek Technologies is proud to announce publication of a peer-reviewed research article by its CEO, Patrick Nemechek D.O. Entitled, ‘Transcutaneous Vagus Nerve Stimulation is Associated with Lower Mechanical Ventilation and Mortality in COVID-19 Patients: An interim Safety Analysis’, the study reveals that transcutaneous vagus nerve stimulation (tVNS) …